Articles
- Page Path
- HOME > J Musculoskelet Trauma > Volume 38(4); 2025 > Article
-
Review Article
- Innovative applications of artificial intelligence in orthopedics focusing on fracture and trauma treatment: a narrative review
-
Chul-Ho Kim
 , Ji Wan Kim
, Ji Wan Kim
-
Journal of Musculoskeletal Trauma 2025;38(4):178-185.
DOI: https://doi.org/10.12671/jmt.2025.00283
Published online: October 24, 2025
Department of Orthopedic Surgery, Asan Medical Center, University of Ulsan College of Medicine, Seoul, Korea
- Correspondence to: Ji Wan Kim Department of Orthopedic Surgery, Asan Medical Center, University of Ulsan College of Medicine, 88 Olympic-ro 43gil, Songpa-gu, Seoul 05505, Korea Tel: +82-2-3010-3530 Email: jaykim@amc.seoul.kr
© 2025 The Korean Orthopaedic Trauma Association
This is an Open Access article distributed under the terms of the Creative Commons Attribution Non-Commercial License (http://creativecommons.org/licenses/by-nc/4.0/) which permits unrestricted noncommercial use, distribution, and reproduction in any medium, provided the original work is properly cited.
- 916 Views
- 2,147,483,661 Download
Abstract
-
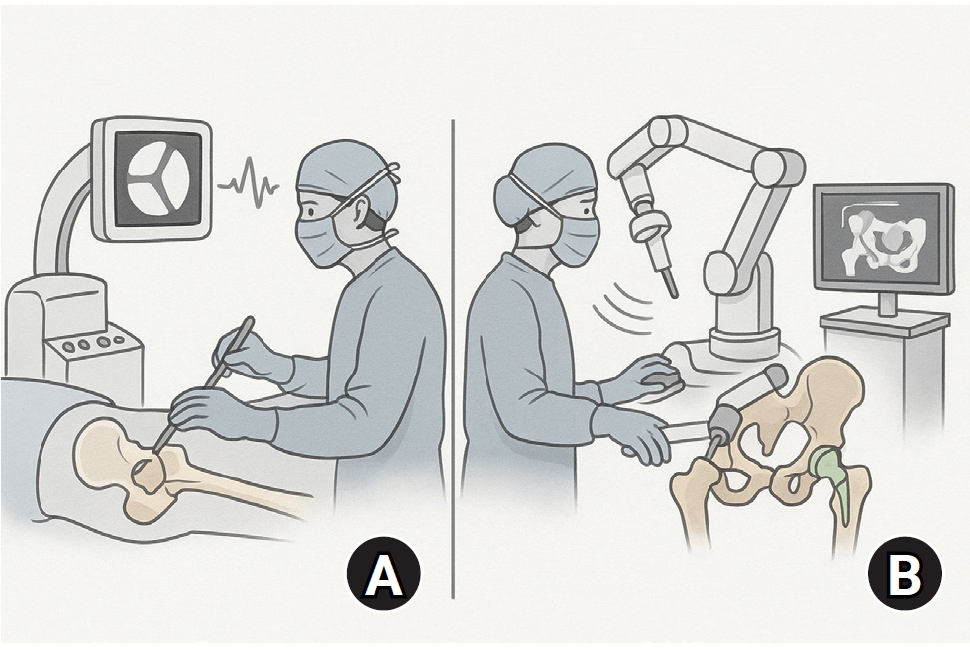
Artificial intelligence (AI) is bringing about transformative changes in orthopedic surgery, with its potential being particularly prominent in the field of fracture and trauma treatment. This review explores the current applications and future prospects of AI-driven surgical planning and simulation, robot and image-based navigation surgery, and image-assisted diagnostic technologies. Robotic assistance in orthopedic surgery, which was initially applied to improve accuracy in component implantation for knee and hip arthroplasty and to achieve high precision in spinal screw placement, has recently expanded its use to include accurate, minimally invasive reduction of pelvic fractures. In diagnostics, AI aids in the early prediction and classification of ambiguous fractures in various anatomical regions—for example, detecting shoulder or hip fractures, identifying incomplete atypical femur fractures, and classifying femoral neck fractures—through X-ray image analysis. This improves diagnostic accuracy and reduces medical costs. However, significant challenges remain, including high initial costs, steep learning curves, a lack of long-term studies, data bias, and ethical concerns. Continued research, interdisciplinary collaboration, and policy support are crucial for the widespread adoption of these technologies.Level of evidence: IV.
Introduction
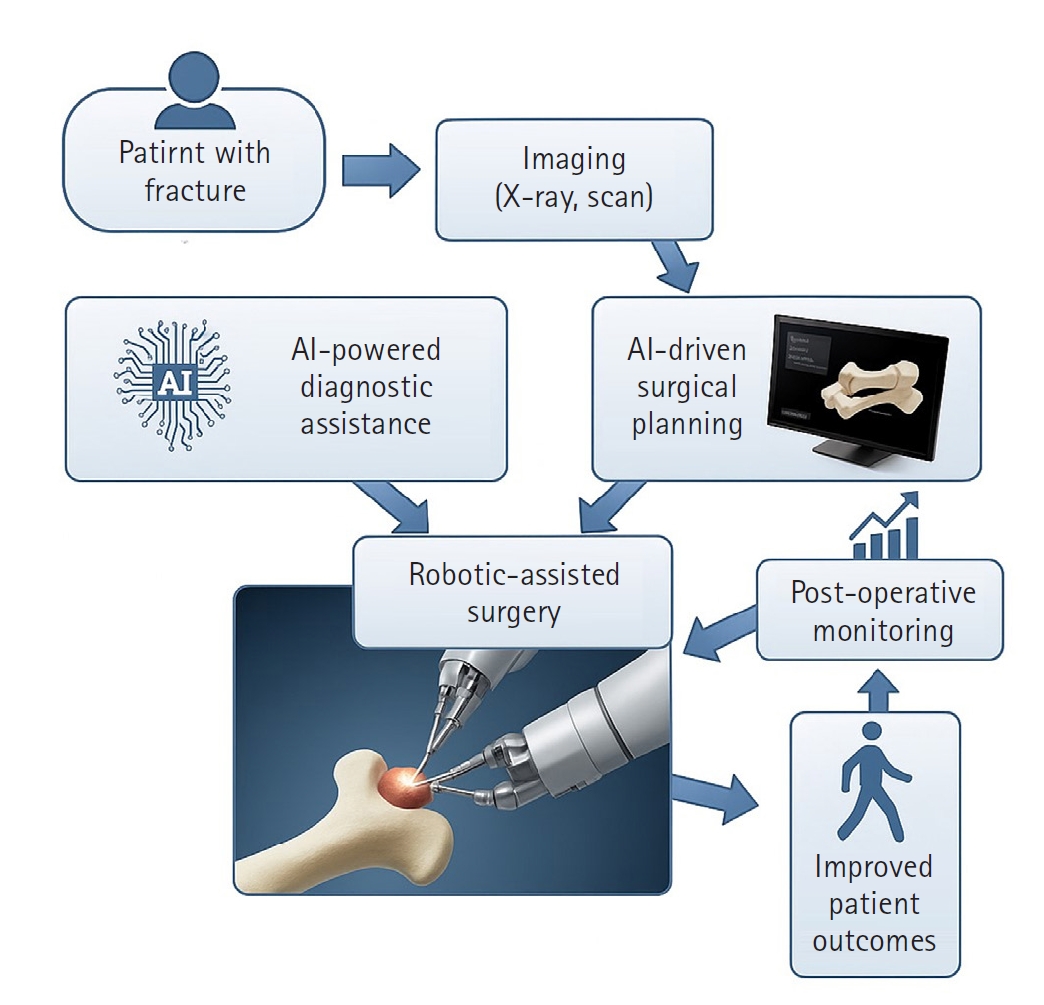
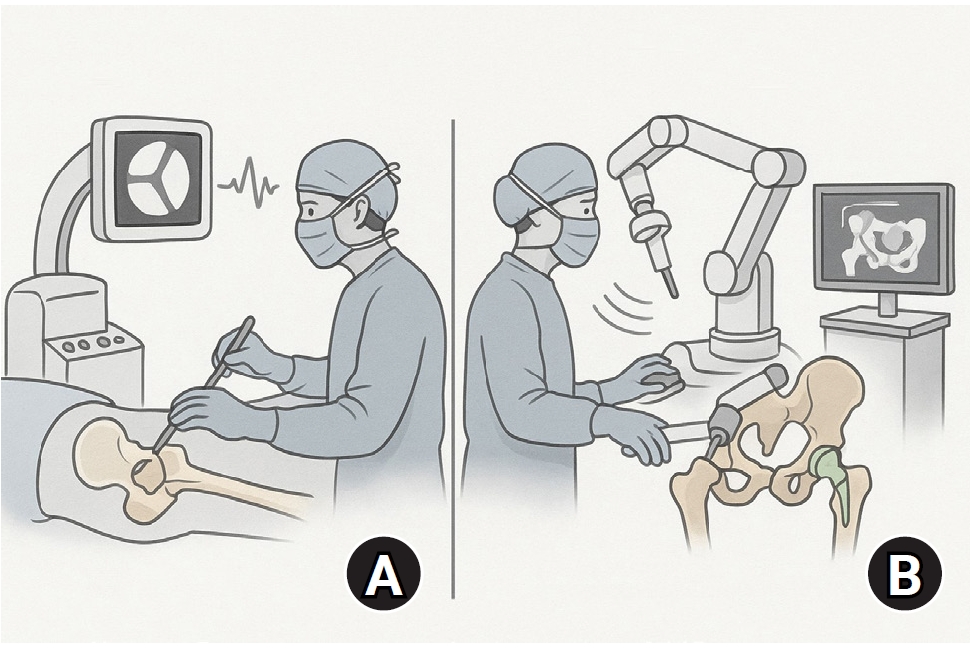
AI-driven surgical planning and robotic-assisted surgery
Image-based assisted diagnostic technologies
Challenges and future outlook
Conclusions
-
Author contribution
Conceptualization: CHK, JWK. Data curation: CHK, JWK. Formal analysis: CHK, JWK. Methodology: CHK, JWK. Investigation: CHK, JWK. Resources: CHK, JWK. Software: CHK, JWK. Supervision: JWK. Validation: CHK, JWK. Project administration: CHK, JWK. Visualization: CHK. Writing-original draft: CHK. Writing-review & editing: JWK. All authors read and approved the final manuscript.
-
Conflict of interests
Ji Wan Kim is a Deputy Editor of this journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
-
Funding
None.
-
Data availability
Not applicable.
-
Acknowledgments
None.
-
Supplementary materials
None.
-
Disclosure of generative AI use
During the preparation of this manuscript, Gemini Pro 2.5 and NotebookLM were used to summarize background literature, assist in language editing, and grammar checking. The authors reviewed and edited the content generated by the AI tool and take full responsibility for the final version of the manuscript. The AI tool was not listed as an author and was used solely as a supportive resource.
Article Information
- 1. Hashimoto DA, Rosman G, Rus D, Meireles OR. Artificial intelligence in surgery: promises and perils. Ann Surg 2018;268:70-6.PubMed
- 2. Hui AT, Alvandi LM, Eleswarapu AS, Fornari ED. Artificial intelligence in modern orthopaedics: current and future applications. JBJS Rev 2022;10.
- 3. Ram PR, Jeyaraman M, Jeyaraman N, Yadav S, Venkatasalam R. Revolutionizing orthopedic healthcare: the role of robotics. Cureus 2023;15:e44820.ArticlePubMedPMC
- 4. Lang JE, Mannava S, Floyd AJ, et al. Robotic systems in orthopaedic surgery. J Bone Joint Surg Br 2011;93:1296-9.ArticlePubMedPDF
- 5. Karthik K, Colegate-Stone T, Dasgupta P, Tavakkolizadeh A, Sinha J. Robotic surgery in trauma and orthopaedics: a systematic review. Bone Joint J 2015;97-B:292-9.PubMed
- 6. Bargar WL, Bauer A, Börner M. Primary and revision total hip replacement using the Robodoc system. Clin Orthop Relat Res 1998;(354):82-91.ArticlePubMed
- 7. Lim SJ, Kim SM, Lim BH, Moon YW, Park YS. Comparison of manual rasping and robotic milling for short metaphyseal-fitting stem implantation in total hip arthroplasty: a cadaveric study. Comput Aided Surg 2013;18:33-40.ArticlePubMed
- 8. Sukovich W, Brink-Danan S, Hardenbrook M. Miniature robotic guidance for pedicle screw placement in posterior spinal fusion: early clinical experience with the SpineAssist. Int J Med Robot 2006;2:114-22.ArticlePubMed
- 9. Xiao X, Wang X, Meng B, Pan X, Zhao H. Comparison of robotic AI-assisted and manual pedicle screw fixation for treating thoracolumbar fractures: a retrospective controlled trial. Front Bioeng Biotechnol 2025;13:1491775.ArticlePubMedPMC
- 10. Jiang B, Pennington Z, Zhu A, et al. Three-dimensional assessment of robot-assisted pedicle screw placement accuracy and instrumentation reliability based on a preplanned trajectory. J Neurosurg Spine 2020;33:519-28.ArticlePubMed
- 11. Mason A, Paulsen R, Babuska JM, et al. The accuracy of pedicle screw placement using intraoperative image guidance systems. J Neurosurg Spine 2014;20:196-203.ArticlePubMed
- 12. Ma M, Wang Z, Ye J, Chen X. Effectiveness of TiRobot-assisted and free-hand percutaneous kyphoplasty via pedicle of vertebra in treatment of osteoporotic vertebral compression fracture of thoracic vertebra. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi 2023;37:1106-12.PubMedPMC
- 13. Wu Z, Dai Y, Zeng Y. Intelligent robot-assisted fracture reduction system for the treatment of unstable pelvic fractures. J Orthop Surg Res 2024;19:271.ArticlePubMedPMCPDF
- 14. Zhao C, Cao Q, Sun X, Wu X, Zhu G, Wang Y. Intelligent robot-assisted minimally invasive reduction system for reduction of unstable pelvic fractures. Injury 2023;54:604-14.ArticlePubMed
- 15. Zhao C, Wang Y, Wu X, Zhu G, Shi S. Design and evaluation of an intelligent reduction robot system for the minimally invasive reduction in pelvic fractures. J Orthop Surg Res 2022;17:205.ArticlePubMedPMCPDF
- 16. Garcia P, Rosen J, Kapoor C, et al. Trauma Pod: a semi-automated telerobotic surgical system. Int J Med Robot 2009;5:136-46.ArticlePubMed
- 17. Lei H, Sheng L, Manyi W, Junqiang W, Wenyong L. A biplanar robot navigation system for the distal locking of intramedullary nails. Int J Med Robot 2010;6:61-5.ArticlePubMedPDF
- 18. Oszwald M, Westphal R, Klepzig D, et al. Robotized access to the medullary cavity for intramedullary nailing of the femur. Technol Health Care 2010;18:173-80.ArticlePubMed
- 19. Garcia JC, Lebailly F, Mantovani G, Mendonca LA, Garcia J, Liverneaux P. Telerobotic manipulation of the brachial plexus. J Reconstr Microsurg 2012;28:491-4.ArticlePubMed
- 20. Mantovani G, Liverneaux P, Garcia JC, Berner SH, Bednar MS, Mohr CJ. Endoscopic exploration and repair of brachial plexus with telerobotic manipulation: a cadaver trial. J Neurosurg 2011;115:659-64.ArticlePubMed
- 21. Lan H, Tan Z, Li KN, Gao JH, Liu TH. Intramedullary nail fixation assisted by orthopaedic robot navigation for intertrochanteric fractures in elderly patients. Orthop Surg 2019;11:255-62.ArticlePubMedPMCPDF
- 22. Lu C, Wei X, Li L, et al. Robot-assisted PFNA surgery improves clinical outcomes in the treatment of unstable femoral intertrochanteric fractures in elderly patients compared with traditional PFNA surgery. Sci Rep 2025;15:3836.ArticlePubMedPMCPDF
- 23. Federer SJ, Jones GG. Artificial intelligence in orthopaedics: a scoping review. PLoS One 2021;16:e0260471.ArticlePubMedPMC
- 24. Eller-Vainicher C, Chiodini I, Santi I, et al. Recognition of morphometric vertebral fractures by artificial neural networks: analysis from GISMO Lombardia database. PLoS One 2011;6:e27277.ArticlePubMedPMC
- 25. Saygılı A, Albayrak S. An efficient and fast computer-aided method for fully automated diagnosis of meniscal tears from magnetic resonance images. Artif Intell Med 2019;97:118-30.ArticlePubMed
- 26. Carballido-Gamio J, Yu A, Wang L, et al. Hip fracture discrimination based on Statistical Multi-parametric Modeling (SMPM). Ann Biomed Eng 2019;47:2199-212.ArticlePubMedPMCPDF
- 27. Nguyen HH, Le DT, Shore-Lorenti C. AFFnet: a deep convolutional neural network for the detection of atypical femur fractures from anteriorposterior radiographs. Bone 2024;187:117215.ArticlePubMed
- 28. Russe MF, Rebmann P, Tran PH. AI-based X-ray fracture analysis of the distal radius: accuracy between representative classification, detection and segmentation deep learning models for clinical practice. BMJ Open 2024;14:e076954.ArticlePubMedPMC
- 29. Hemanth Kumar M, Karthika M, Saianiruth M, et al. A deep learning-based ensemble system for automated shoulder fracture detection in clinical radiographs [Preprint]. Posted 2025 Jul 17. arXiv 2507.13408. https://arxiv.org/abs/2507.13408.
- 30. Kazley JM, Banerjee S, Abousayed MM, Rosenbaum AJ. Classifications in brief: garden classification of femoral neck fractures. Clin Orthop Relat Res 2018;476:441-5.ArticlePubMedPMC
- 31. Zlowodzki M, Bhandari M, Keel M, Hanson BP, Schemitsch E. Perception of garden's classification for femoral neck fractures: an international survey of 298 orthopaedic trauma surgeons. Arch Orthop Trauma Surg 2005;125:503-5.ArticlePubMedPDF
- 32. Xing P, Zhang L, Wang T, Wang L, Xing W, Wang W. A deep learning algorithm that aids visualization of femoral neck fractures and improves physician training. Injury 2024;55:111997.ArticlePubMed
- 33. Cha Y, Kim JT, Park CH, Kim JW, Lee SY, Yoo JI. Artificial intelligence, machine learning on diagnosis, classification of hip fracture. systematic review. J Orthop Surg Res 2022;17:520.PubMedPMC
- 34. Sato Y, Takegami Y, Asamoto T, et al. Artificial intelligence improves the accuracy of residents in the diagnosis of hip fractures: a multicenter study. BMC Musculoskelet Disord 2021;22:407.PubMedPMC
- 35. Murphy EA, Ehrhardt B, Gregson CL, et al. Machine learning outperforms clinical experts in classification of hip fractures. Sci Rep 2022;12:2058.ArticlePubMedPMCPDF
- 36. Yoon SJ, Hyong Kim T, Joo SB, Eel Oh S. Automatic multi-class intertrochanteric femur fracture detection from CT images based on AO/OTA classification using faster R-CNN-BO method. J Appl Biomed 2020;18:97-105.ArticlePubMed
- 37. Adams M, Chen W, Holcdorf D, McCusker MW, Howe PD, Gaillard F. Computer vs human: deep learning versus perceptual training for the detection of neck of femur fractures. J Med Imaging Radiat Oncol 2019;63:27-32.ArticlePubMedPDF
- 38. Urakawa T, Tanaka Y, Goto S, Matsuzawa H, Watanabe K, Endo N. Detecting intertrochanteric hip fractures with orthopedist-level accuracy using a deep convolutional neural network. Skeletal Radiol 2019;48:239-44.ArticlePubMedPDF
- 39. Cheng CT, Ho TY, Lee TY, et al. Application of a deep learning algorithm for detection and visualization of hip fractures on plain pelvic radiographs. Eur Radiol 2019;29:5469-77.ArticlePubMedPMCPDF
- 40. Krogue JD, Cheng KV, Hwang KM, et al. Automatic hip fracture identification and functional subclassification with deep learning. Radiol Artif Intell 2020;2:e190023.ArticlePubMedPMC
- 41. Yu JS, Yu SM, Erdal BS, et al. Detection and localisation of hip fractures on anteroposterior radiographs with artificial intelligence: proof of concept. Clin Radiol 2020;75:237.e1-9.ArticlePubMed
- 42. Mutasa S, Varada S, Goel A, Wong TT, Rasiej MJ. Advanced deep learning techniques applied to automated femoral neck fracture detection and classification. J Digit Imaging 2020;33:1209-17.ArticlePubMedPMCPDF
- 43. Beyaz S, Açıcı K, Sümer E. Femoral neck fracture detection in X-ray images using deep learning and genetic algorithm approaches. Jt Dis Relat Surg 2020;31:175-83.ArticlePubMedPMC
- 44. Mawatari T, Hayashida Y, Katsuragawa S, et al. The effect of deep convolutional neural networks on radiologists' performance in the detection of hip fractures on digital pelvic radiographs. Eur J Radiol 2020;130:109188.ArticlePubMed
- 45. Yamada Y, Maki S, Kishida S, et al. Automated classification of hip fractures using deep convolutional neural networks with orthopedic surgeon-level accuracy: ensemble decision-making with antero-posterior and lateral radiographs. Acta Orthop 2020;91:699-704.ArticlePubMedPMC
- 46. Cheng CT, Chen CC, Cheng FJ, et al. A human-algorithm integration system for hip fracture detection on plain radiography: system development and validation study. JMIR Med Inform 2020;8:e19416.ArticlePubMedPMC
- 47. Bae J, Yu S, Oh J, et al. External validation of deep learning algorithm for detecting and visualizing femoral neck fracture including displaced and non-displaced fracture on plain X-ray. J Digit Imaging 2021;34:1099-109.ArticlePubMedPMCPDF
- 48. Yang YL, Zhou DS, He JL. Comparison of isocentric C-arm 3-dimensional navigation and conventional fluoroscopy for C1 lateral mass and C2 pedicle screw placement for atlantoaxial instability. J Spinal Disord Tech 2013;26:127-34.ArticlePubMed
- 49. Moon SW, Kim JW. Usefulness of intraoperative three-dimensional imaging in fracture surgery: a prospective study. J Orthop Sci 2014;19:125-31.ArticlePubMed
- 50. Hammerle D, Osterhoff G, Allemann F, Werner CM. Comparison of intraoperative 2D vs. 3D imaging in open reduction and fixation of distal radius fractures. Eur J Trauma Emerg Surg 2020;46:557-63.ArticlePubMedPDF
- 51. Khoury A, Whyne CM, Daly M, et al. Intraoperative cone-beam CT for correction of periaxial malrotation of the femoral shaft: a surface-matching approach. Med Phys 2007;34:1380-7.ArticlePubMedPDF
- 52. Franke J, von Recum J, Suda AJ, Grutzner PA, Wendl K. Intraoperative three-dimensional imaging in the treatment of acute unstable syndesmotic injuries. J Bone Joint Surg Am 2012;94:1386-90.ArticlePubMed
- 53. Franke J, Wendl K, Suda AJ, Giese T, Grützner PA, von Recum J. Intraoperative three-dimensional imaging in the treatment of calcaneal fractures. J Bone Joint Surg Am 2014;96:e72.ArticlePubMed
- 54. Dagnino G, Georgilas I, Morad S, et al. Image-guided surgical robotic system for percutaneous reduction of joint fractures. Ann Biomed Eng 2017;45:2648-62.ArticlePDF
- 55. Sato Y, Yamamoto N, Inagaki N, et al. Deep learning for bone mineral density and t-score prediction from chest X-rays: a multicenter study. Biomedicines 2022;10:2323.Article
- 56. Zhou L, Nguyen T, Choi S, Yoon J. U-net-based deep learning hybrid model: research and evaluation for precise prediction of spinal bone density on abdominal radiographs. Bioengineering (Basel) 2025;12:385.
- 57. Choi W, Kim CH, Yoo H, Yun HR, Kim DW, KiM JW. Development and validation of a reliable method for automated measurements of psoas muscle volume in CT scans using deep learning-based segmentation: a cross-sectional study. BMJ Open 2024;14:e079417.Article
- 58. Swank ML, Alkire M, Conditt M, Lonner JH. Technology and cost-effectiveness in knee arthroplasty: computer navigation and robotics. Am J Orthop (Belle Mead NJ) 2009;38(2 Suppl):32-6.
- 59. Pearle AD, O'Loughlin PF, Kendoff DO. Robot-assisted unicompartmental knee arthroplasty. J Arthroplasty 2010;25:230-7.Article
- 60. Elswick CM, Strong MJ, Joseph JR, Saadeh Y, Oppenlander M, Park P. Robotic-assisted spinal surgery: current generation instrumentation and new applications. Neurosurg Clin N Am 2020;31:103-10.
- 61. Gao S, Lv Z, Fang H. Robot-assisted and conventional freehand pedicle screw placement: a systematic review and meta-analysis of randomized controlled trials. Eur Spine J 2018;27:921-30.ArticlePDF
References
Figure & Data
REFERENCES
Citations


 E-submission
E-submission KOTA
KOTA TOTA
TOTA TOTS
TOTS
 ePub Link
ePub Link Cite
Cite